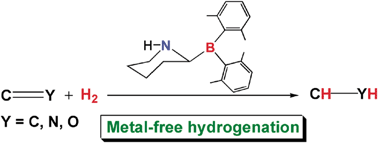
A computational experiment to study hydrogenations of various unsaturated compounds catalyzed by a rationally designed metal-free catalyst†
Abstract
Metal-free ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (4)),
CH2 (4)), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)OSiMe3 (5)),
C(Me)OSiMe3 (5)), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe (6) and Ph(Me)C
NMe (6) and Ph(Me)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe (7)), and
NMe (7)), and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (9)). The energetic results predicted at the M05-2X(IEFPCM,
O (9)). The energetic results predicted at the M05-2X(IEFPCM,

 Please wait while we load your content...
Please wait while we load your content...