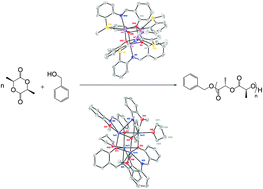
Synthesis, characterization and catalytic activity of lithium and sodium iminophenoxide complexes towards ring-opening polymerization of l-lactide†
Abstract
A series of lithium and sodium iminophenoxide complexes have been successfully synthesized and characterized by

 Please wait while we load your content...
Please wait while we load your content...