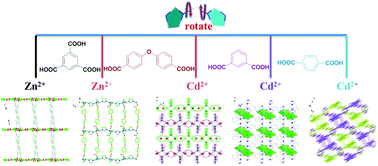
Five new ZnII/CdII coordination polymers constructed from di(1H-imidazol-1-yl)methane (L) mixed with different auxiliary carboxylic acid ligands formulated as [Zn(L)(H2L1111)2·(H2O)0.2]n (1), {[Zn(L)(L222)]·H2O}n (2), {[Cd2(L)2(L222)2]·2H2O}n (3), {[Cd(L)(L33)]·H2O}n (4) and [Cd(L)(L44)]n (5) (H3L1111 = 1,3,5-benzenetricarboxylic acid, H2L222 = 4,4′-oxybis(benzoic acid), H2L33 = m-phthalic acid and H2L44 = p-phthalic acid) have been synthesized under hydrothermal conditions and structurally characterized. Four related auxiliary carboxylic acids were chosen to examine the influences on the construction of these coordination frameworks with distinct dimensionality and connectivity. The coordination arrays of 1–5 vary from 1D zigzag chain for 1, 2D (4,4) layer for 2–4, to 2-fold interpenetrated 3D coordination network with the α-Po topology for 5. The thermal and photoluminescence properties of complexes 1–5 in the solid state have also been investigated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...