Copper(i) and nickel(ii) complexes with 1 : 1 vs. 1 : 2 coordination of ferrocenyl hydrazone ligands: Do the geometry and composition of complexes affect DNA binding/cleavage, protein binding, antioxidant and cytotoxic activities?†
Abstract
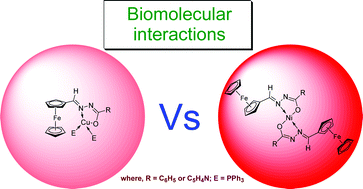
A new series of geometrically different complexes containing ferrocenyl hydrazone ligands were synthesised by reacting suitable precursor complex [MCl2(PPh3)2] with the ligands HL1 or HL2 (where M = Cu(II) or Ni(II); HL1 = [Cp2Fe(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH –CO– C6H5)] (1) and HL2 = [Cp2Fe(CH
N–NH –CO– C6H5)] (1) and HL2 = [Cp2Fe(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–NH–CO–C5H4N)]) (2). The new complexes of the composition [Cu(L1)(PPh3)2], (3) [Cu(L2)(PPh3)2] (4), [Ni(L1)2] (5) and [Ni(L2)2] (6) were characterised by various spectral studies. Among them, complexes 3 and 5 characterised by single crystal X-ray diffraction showed a distorted tetrahedral structure for the former with 1 : 1 metal–ligand stoichiometry, but a distorted square planar geometry with 1 : 2 metal–ligand stoichiometry in the case of the latter. Systematic biological investigations like DNA binding, DNA cleavage, protein binding, free radical scavenging and cytotoxicity activities were carried out using all the synthesised compounds and the results obtained were explained on the basis of structure–activity relationships. The binding constant (Kb) values of the synthesised compounds are found to be in the order of magnitude 103–105 M−1 and also they exhibit significant cleavage of supercoiled (SC) pUC19 DNA in the presence of H2O2 as co-oxidant. The conformational changes of bovine serum albumin (BSA) upon binding with the above complexes were also studied. In addition, concentration dependent free radical scavenging potential of all the synthesised compounds (1–6) was also carried out under in vitro conditions. Assays on the cytotoxicity of the above complexes against HeLa and A431 tumor cells and NIH 3T3 normal cells were also carried out.
N–NH–CO–C5H4N)]) (2). The new complexes of the composition [Cu(L1)(PPh3)2], (3) [Cu(L2)(PPh3)2] (4), [Ni(L1)2] (5) and [Ni(L2)2] (6) were characterised by various spectral studies. Among them, complexes 3 and 5 characterised by single crystal X-ray diffraction showed a distorted tetrahedral structure for the former with 1 : 1 metal–ligand stoichiometry, but a distorted square planar geometry with 1 : 2 metal–ligand stoichiometry in the case of the latter. Systematic biological investigations like DNA binding, DNA cleavage, protein binding, free radical scavenging and cytotoxicity activities were carried out using all the synthesised compounds and the results obtained were explained on the basis of structure–activity relationships. The binding constant (Kb) values of the synthesised compounds are found to be in the order of magnitude 103–105 M−1 and also they exhibit significant cleavage of supercoiled (SC) pUC19 DNA in the presence of H2O2 as co-oxidant. The conformational changes of bovine serum albumin (BSA) upon binding with the above complexes were also studied. In addition, concentration dependent free radical scavenging potential of all the synthesised compounds (1–6) was also carried out under in vitro conditions. Assays on the cytotoxicity of the above complexes against HeLa and A431 tumor cells and NIH 3T3 normal cells were also carried out.

 Please wait while we load your content...
Please wait while we load your content...