The ring–chain tautomeric equilibria of selenium macrocyclic compounds: the isolation of the ring tautomer†
Abstract
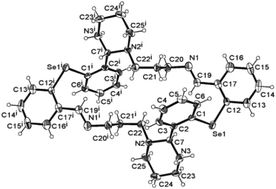
A broadening of the investigation of the ring–chain tautomeric process of N-substituted 1,3-X,N-heterocycles (X = O, S, NR) to Se containing macrocyclic compounds allowed the isolation and structurally solid state characterization of the cyclic tautomer 7, which due to the length of the aliphatic chain, is able to form a stable six-membered ring (6-endo-trig). The theoretical calculations based on the DFT method (Gaussian 03 software package) also support the fact that tautomer 7 is more stable than the chain tautomer 6. Thus, based on the ring–chain tautomerism of the

 Please wait while we load your content...
Please wait while we load your content...