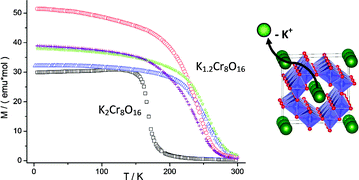
The half-metallic ferromagnet K2Cr8O16 with the hollandite structure has been chemically modified using soft chemistry methods to increase the average oxidation state of chromium. The synthesis of the parent material has been performed under high pressure/high temperature conditions. Following this, different redox reactions have been carried out on K2Cr8O16. Oxidation to obtain potassium-de-inserted derivatives, K2−xCr8O16 (0 ≤ x ≤ 1), has been investigated with electrochemical methods, while the synthesis of sizeable amounts was achieved chemically by using nitrosonium tetrafluoroborate as a highly oxidizing agent. The maximum amount of extracted K ions corresponds to x = 0.8. Upon oxidation the hollandite structure is maintained and the products keep high crystallinity. The de-insertion of potassium changes the Cr3+/Cr4+ ratio, and therefore the magnetic properties. Interestingly, the Curie temperature increases from ca. 175 K to 250 K, getting therefore closer to room temperature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...