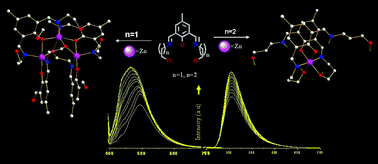
The phenoxido and alkoxido bridged neutral Zn3 complex [Zn3(μ-H2bemp)2(μ3-emp)2] (1), with an angular Zn3(μ-OPh)2(μ-OEt)2 core and capping nitrogen donors, was synthesized via simultaneous chelation-cum-bridging of the parent and hydrolysed ligands. Zinc(II) coordination triggered the solution phase imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond hydrolysis of H3bemp (2,6-bis-[(2-hydroxyethylimino)methyl]-4-methylphenol) and yielded the unexpected angular trinuclear Zn(II) complex 1, having structural similarity with the Zn3 active site of P1 nuclease. H3bemp also displays a zinc(II) selective chelation-enhanced fluorescence response from strong metal ion coordination. Complexation of zinc(II) with H3bpmp (2,6-bis-[(3-hydroxypropylimino)methyl]-4-methylphenol), a close analogue of H3bemp, instead provides only mononuclear [Zn(H2bpmpHN)2](ClO4)2·2H2O (2·2H2O) (HN is the proton attached to an imine nitrogen atom) of two zwitterionic ligands, generated through a kind of coordination driven acid–base reaction, without showing any aggregation reaction. As the sole metal–organic precursor, both the complexes under pyrolytic conditions give ZnO nano structures of two morphologies.
N) bond hydrolysis of H3bemp (2,6-bis-[(2-hydroxyethylimino)methyl]-4-methylphenol) and yielded the unexpected angular trinuclear Zn(II) complex 1, having structural similarity with the Zn3 active site of P1 nuclease. H3bemp also displays a zinc(II) selective chelation-enhanced fluorescence response from strong metal ion coordination. Complexation of zinc(II) with H3bpmp (2,6-bis-[(3-hydroxypropylimino)methyl]-4-methylphenol), a close analogue of H3bemp, instead provides only mononuclear [Zn(H2bpmpHN)2](ClO4)2·2H2O (2·2H2O) (HN is the proton attached to an imine nitrogen atom) of two zwitterionic ligands, generated through a kind of coordination driven acid–base reaction, without showing any aggregation reaction. As the sole metal–organic precursor, both the complexes under pyrolytic conditions give ZnO nano structures of two morphologies.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) bond
N) bond 

 Please wait while we load your content...
Please wait while we load your content...