The reaction of tBu(C6H4O2)P, with the borane B(C6F5)3 gives rise to NMR data consistent with the formation of the classical Lewis acid–base adduct tBu(C6H4O2)P(B(C6F5)3) (1). In contrast, the NMR data for the corresponding reactions of tBu(C20H12O2)P and Cl(C20H12O2)P with B(C6F5)3 were consistent with the presence of equilibria between free phosphine and borane and the corresponding adducts. Nonetheless, in each case, the adducts tBu(C20H12O2)P(B(C6F5)3) (2) and Cl(C20H12O2)P(B(C6F5)3) (3) were isolable. The species 1 reacts with PhCCH to give the new species tBu(C6H4O2)P(Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)3 (4) in near quantitative yield. In an analogous fashion, the addition of PhCCH to solutions of the phosphinestBu(C20H12O2)P, tBuPCl2 and (C6H3(2,4-tBu2)O)3P each with an equivalent of B(C6F5)3 gave rise to L(Ph)C
CHB(C6F5)3 (4) in near quantitative yield. In an analogous fashion, the addition of PhCCH to solutions of the phosphinestBu(C20H12O2)P, tBuPCl2 and (C6H3(2,4-tBu2)O)3P each with an equivalent of B(C6F5)3 gave rise to L(Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)3 (L = tBu(C20H12O2)P5, tBuPCl26 and (C6H3(2,4-tBu2)O)3P 7). X-Ray data for 1, 2, 6 and 7 are presented. The implications of these findings are considered.
CHB(C6F5)3 (L = tBu(C20H12O2)P5, tBuPCl26 and (C6H3(2,4-tBu2)O)3P 7). X-Ray data for 1, 2, 6 and 7 are presented. The implications of these findings are considered.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)3 (4) in near quantitative yield. In an analogous fashion, the addition of PhCCH to solutions of the phosphinestBu(C20H12O2)P, tBuPCl2 and (C6H3(2,4-tBu2)O)3P each with an equivalent of B(C6F5)3 gave rise to L(Ph)C
CHB(C6F5)3 (4) in near quantitative yield. In an analogous fashion, the addition of PhCCH to solutions of the phosphinestBu(C20H12O2)P, tBuPCl2 and (C6H3(2,4-tBu2)O)3P each with an equivalent of B(C6F5)3 gave rise to L(Ph)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHB(C6F5)3 (L = tBu(C20H12O2)P5, tBuPCl26 and (C6H3(2,4-tBu2)O)3P 7). X-Ray data for 1, 2, 6 and 7 are presented. The implications of these findings are considered.
CHB(C6F5)3 (L = tBu(C20H12O2)P5, tBuPCl26 and (C6H3(2,4-tBu2)O)3P 7). X-Ray data for 1, 2, 6 and 7 are presented. The implications of these findings are considered.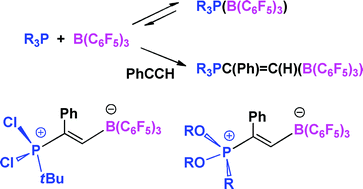

 Please wait while we load your content...
Please wait while we load your content...