Transformations of aryl isothiocyanates on tetraphosphine tungsten complexes and reactivity of the resulting dithiocarbonimidate ligand†
Abstract
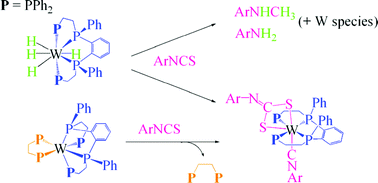
Treatment of [WH4(κ4-P4)] (3: P4 = meso-o-C6H4(PPhCH2CH2PPh2)2) with aryl isothiocyanate ArNCS at 50 °C afforded the dithiocarbonimidate-isocyanide complex [W(κ2-S2CNAr)(CNAr)(κ4-P4)] (4) in moderate yields. The reaction also produced ArNHCH3 and a small amount of ArNH2. The yield of the hydrodesulfurization product ArNHCH3 increased when the reaction was conducted under H2 (up to 0.65 equiv. to 3 for Ar = p-MeC6H4 (Tol)). Complex 4 was proposed to be formed via reductive disproportionation of two ArNCS molecules on a zero-valent W species generated by dissociation of H2 from 3. The reaction of W(0) complex [W(dppe)(κ4-P4)] (

 Please wait while we load your content...
Please wait while we load your content...