Modular ligand variation in calcium bisimidazoline complexes: effects on ligand redistribution and hydroamination catalysis†
Abstract
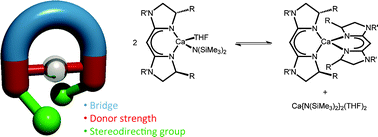
A series of calcium complexes supported by chiral bisimidazoline
- This article is part of the themed collection: d0 organometallics in catalysis

 Please wait while we load your content...
Please wait while we load your content...