Mononuclear and dinuclear palladium and nickel complexes of phosphinimine-based tridentate ligands†
Abstract
The tridentate bis-phosphinimine ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)21, HN(1,2-C2H4N
PPh3)21, HN(1,2-C2H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3)2 (R = Ph 2, iPr 3), MeN(1,2-C2H4N
PR3)2 (R = Ph 2, iPr 3), MeN(1,2-C2H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)24 and HN(1,2-C6H4N
PPh3)24 and HN(1,2-C6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)25 were prepared. Employing these
PPh3)25 were prepared. Employing these ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2PdCl26, RN(1,2-CH2CH2N
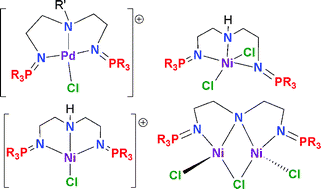
PPh3)2PdCl26, RN(1,2-CH2CH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2PdCl][Cl] (R = H 7, Me 8), [HN(1,2-CH2CH2N
PPh3)2PdCl][Cl] (R = H 7, Me 8), [HN(1,2-CH2CH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PiPr3)2PdCl][Cl] 9, [MeN(1,2-CH2CH2N
PiPr3)2PdCl][Cl] 9, [MeN(1,2-CH2CH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2PdCl][PF6] 10, [HN(1,2-CH2CH2N
PPh3)2PdCl][PF6] 10, [HN(1,2-CH2CH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2NiCl2] 11, [HN(1,2-CH2CH2N
PPh3)2NiCl2] 11, [HN(1,2-CH2CH2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3)2NiCl][X] (X = Cl, R = iPr 12, X = PF6, R = Ph 13, iPr 14), and [HN(1,2-C6H4N
PR3)2NiCl][X] (X = Cl, R = iPr 12, X = PF6, R = Ph 13, iPr 14), and [HN(1,2-C6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2Ni(MeCN)2][BF4]Cl 15 were prepared and characterized. While the ether-bis-phosphinimine
PPh3)2Ni(MeCN)2][BF4]Cl 15 were prepared and characterized. While the ether-bis-phosphinimine ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3)2Ni2Cl3 (R = Ph 16, iPr 17) and N(1,2-C6H4N
PR3)2Ni2Cl3 (R = Ph 16, iPr 17) and N(1,2-C6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3)2Ni2Cl318. The precise nature of a number of these complexes were crystallographically characterized.
PPh3)2Ni2Cl318. The precise nature of a number of these complexes were crystallographically characterized.

 Please wait while we load your content...
Please wait while we load your content...