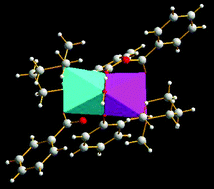
Two new nickel(II) complexes [Ni2L2(PhCOO)2(H2O)] (1), [Ni2L2(PhCH2COO)2(H2O)] (2) have been synthesized using a tridentate Schiff base ligand, HL (2-[(3-dimethylamino-propylimino)-methyl]-phenol) and the carboxylate monoanions, benzoate and phenylacetate, respectively. The complexes have been characterized by spectral analysis, variable temperature magnetic susceptibility measurement and crystal structure analysis. The structural analyses reveal that both complexes are dinuclear in which the distorted octahedral Ni2+ ions share a face, bridged by one water molecule and two μ2-phenoxo oxygen atoms. A monodentate benzoate or phenylacetate anion and two nitrogen atoms of the chelating deprotonated Schiff base (L) complete the hexa-coordination around the metal ion. Variable-temperature magnetic susceptibility studies indicate the presence of dominant ferromagnetic exchange coupling in complexes 1 and 2 with J values of 11.1(2) and 10.9(2) cm−1 respectively. An attempt has been made to rationalize the observed magneto-structural behavior considering the importance of the additional water bridge in the present two complexes and also in other similar species.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...