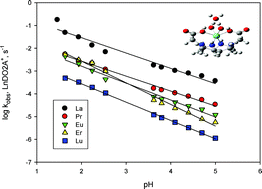
The [H+]-catalyzed dissociation rate constants of several trivalent lanthanide (Ln) complexes of 1,4,7,10-tetraazacyclododecane-1,7-diacetic acid (LnDO2A+, Ln = La, Pr, Eu, Er and Lu) have been determined in two pH ranges: 3.73–5.11 and 1.75–2.65 at four different temperatures (19–41.0 °C) in aqueous media at a constant ionic strength of 0.1 mol dm−3 (LiClO4). For the study in the higher pH range, i.e. pH 3.73–5.11, copper(II) ion was used as the scavenger for the free ligand DO2A in acetate/acetic acid buffer medium. The rates of Ln(III) complex dissociation have been found to be independent of [Cu2+] and all the Ln(III) complexes studied show [H+]-dependence at low acid concentrations but become [H+]-independent at high acid concentrations. Influence of the acetate ion content in the buffer on the dissociation rate has also been investigated and all the complexes exhibit a first-order dependence on [Acetate]. The dissociation reactions follow the rate law: kobs = kAc[Acetate] + K′klim[H+]/(1 + K′[H+]) where kAC is the dissociation rate constant for the [Acetate]-dependent pathway, klim is the limiting rate constant, and K′ is the equilibrium constant for the reaction LnDO2A+ + H+ ⇔ LnDO2AH2+. In the lower pH range, i.e. pH 1.75–2.65, the dye indicator, cresol red, was used to monitor the dissociation rate, and all the Ln(III) complexes also show [H+]-dependence dissociation pathways but without the rate saturation observed at higher pH range. The dissociation reactions follow the simple rate law: kobs = kH[H+], where kH is the dissociation rate constant for the pathway involving monoprotonated species. The absence of an [H+]-independent pathway in both pH ranges indicates that LnDO2A+ complexes are kinetically rather inert. The obtained kAC values follow the order: LaDO2A+ > PrDO2A+ > EuDO2A+ > ErDO2A+ > LuDO2A+, whereas the klim and kH values follow the order: LaDO2A+ > PrDO2A+ > ErDO2A+ > EuDO2A+ > LuDO2A+, mostly consistent with their thermodynamic stability order, i.e. the more thermodynamically stable the more kinetically inert. In both pH ranges, activation parameters, ΔH*, ΔS* and ΔG*, for both acetate-dependent and proton-catalyzed dissociation pathways have been obtained for most of the La(III), Pr(III), Eu(III), Er(III) and Lu(III) complexes, from the temperature dependence measurements of the rate constants in the 19–41 °C range. An isokinetic (linear) relationship is found between ΔH* and ΔS* values, which supports a common reaction mechanism.
 Please wait while we load your content...
Please wait while we load your content...