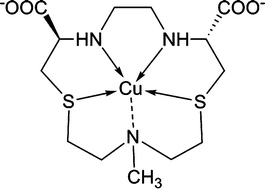
We investigated, both in the solid state and in aqueous solution, the coordination environment and stability behavior of four macrocyclic ligands (three N2S2 and one N3S2) and of the corresponding Cu(II) complexes. The structural characterization in the solid state of the copper derivatives was performed by X-Ray Absorption Spectroscopy. Copper is found to be 4-fold coordinated with a CuN2S2 environment with different Cu–S distances depending on the size of the macrocyclic ring. The EXAFS technique has indicated that nitrogen and sulfur atoms are more preferable to oxygen atoms as donor systems, without the evidence of coordination of the carboxylic moieties to copper in the first shell. The joint EXAFS and XANES study of the copper(II) complex with the N3S2 ligand confirms the 4-fold coordination with an additional, long Cu–N interaction. The Cu2+ complexation constants for one ligand were determined in aqueous solution. The results indicate that the species [CuL], although isolated in the solid state, is not the most abundant at the pH of blood serum. Instead, at pH 7.4 the protonated [Cu(HL)]+ species was found to be the most relevant. The behaviour of the copper complexes in the presence of the strong copper chelating bioagent human serum albumin was also examined in order to gain information on the stability of these compounds in biological fluids.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...