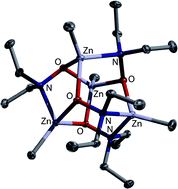
The reactions of zinc dialkyls, R2Zn (1a–d; R = Me (a), Et (b), iPr (c) and tBu (d)), with N,N-dialkylhydroxylamines, HO–NR′2 (2a–c; R′ = Me (a), Et (b) and iPr (c)), afford organozinc hydroxylamides under alkane extrusion. Species of different nuclearity are observed, depending on the hydroxylamine 2 employed. The smaller 2a and 2b give pentanuclear complexes of the general formula Zn(RZn)4O–NR′2)6 (R = Me, Et, iPr and tBu; R′ = Me and Et), whereas the derivatives of 2c are tetramers of the general formula (RZn)4(O–NR′2)4 (R = Me, Et and iPr; R′ = iPr) as governed by bulk issues about the N-donor. Due to the ability of the double-donor unit O–NR2 to change its bridging mode, two coordination isomers exist for both types of compounds. The pentanuclear species crystallise either in a heterofenestrane or an octahedroid motif. For these species, the central Zn atom exhibits either coordination number 4 or 6; in solution, a rapid change between coordination isomers is observed. Due to the absence of a central Zn atom in the tetranuclear species, these aggregate in heterocubane geometries or such derived thereof. They display the O–N units in either κ3O or κ2O;κ1N mode. The tetranuclear species are also yielded with the less sterically encumbered precursors under thermodynamic conditions (i.e. reflux), as exemplified by the reaction of Me2Zn (1a) with HO–NEt2 (2b). They are non-dynamic in solution, showing that a central cation is mandatory for the fluxional behaviour observed for the pentanuclear derivatives. DFT studies on the O–NMe2 series reveal that the relative energies of the pentazinc isomers become more similar with increasing RZn group size; possible conversions of these to their tetrazinc counterparts were also scrutinised. Two κ3O-bridged degradation products of hydroxylamide complexes could be structurally characterised. They were formed either by partial product hydrolysis, or by in situ oxygenation of the starting zinc dialkyl.

 Please wait while we load your content...
Please wait while we load your content...