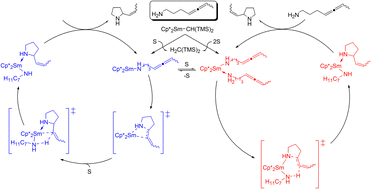
The present mechanistic study comprehensively explores alternative scenarios for activation of the amine-linked allene C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkage toward nucleophilic amido attack in the intramolecular hydroamination of a prototypical 1,3-disubstituted aminoallene by a well-characterised samarocene–amido catalyst. Firstly, the non-insertive mechanism by Scott featuring C–N ring closure with concomitant amino proton delivery onto the allene unit has been explored and its key features have been defined. This scenario has been compared and contrasted with the classical stepwise Ln–N σ-bond insertive mechanism that involves rapid substrate association/dissociation equilibria for the Ln–amido–substrate resting state and also for Ln–azacycle intermediates, facile and reversible exocyclic ring closure through migratory allene insertion into the Ln–N amido σ-bond, linked to turnover-limiting Ln–C azacycle aminolysis. The Ln–N σ-bond insertive mechanism prevails for the studied intramolecular hydroamination of 4,5-heptadien-1-ylamine 1 by [Cp*2Sm–CH(TMS)2] starting material 2. The following aspects are in support of this mechanism: 1) the derived rate law is consistent with the observed empirical rate law; 2) the assessed effective barrier for turnover-limiting aminolysis does agree reasonably well with empirically determined Eyring parameters; 3) the ring-tether double bond selectivity is consistently elucidated. On the other hand, this study reveals that the non-insertive mechanism, which features a prohibitively large barrier, is unachievable. Spatial demands around the lanthanide centre effect the two mechanisms differently. A sufficiently accessible lanthanide is a crucial requirement of the Ln–N σ-bond insertive mechanism and enhanced encumbrance renders the cyclisation step less accessible kinetically. This contrasts with the non-insertive mechanism, where greater lanthanide protection has a rather modest influence. The present study indicates that the non-insertive mechanism would prevail if the lanthanide centre were to be protected effectively against C
C linkage toward nucleophilic amido attack in the intramolecular hydroamination of a prototypical 1,3-disubstituted aminoallene by a well-characterised samarocene–amido catalyst. Firstly, the non-insertive mechanism by Scott featuring C–N ring closure with concomitant amino proton delivery onto the allene unit has been explored and its key features have been defined. This scenario has been compared and contrasted with the classical stepwise Ln–N σ-bond insertive mechanism that involves rapid substrate association/dissociation equilibria for the Ln–amido–substrate resting state and also for Ln–azacycle intermediates, facile and reversible exocyclic ring closure through migratory allene insertion into the Ln–N amido σ-bond, linked to turnover-limiting Ln–C azacycle aminolysis. The Ln–N σ-bond insertive mechanism prevails for the studied intramolecular hydroamination of 4,5-heptadien-1-ylamine 1 by [Cp*2Sm–CH(TMS)2] starting material 2. The following aspects are in support of this mechanism: 1) the derived rate law is consistent with the observed empirical rate law; 2) the assessed effective barrier for turnover-limiting aminolysis does agree reasonably well with empirically determined Eyring parameters; 3) the ring-tether double bond selectivity is consistently elucidated. On the other hand, this study reveals that the non-insertive mechanism, which features a prohibitively large barrier, is unachievable. Spatial demands around the lanthanide centre effect the two mechanisms differently. A sufficiently accessible lanthanide is a crucial requirement of the Ln–N σ-bond insertive mechanism and enhanced encumbrance renders the cyclisation step less accessible kinetically. This contrasts with the non-insertive mechanism, where greater lanthanide protection has a rather modest influence. The present study indicates that the non-insertive mechanism would prevail if the lanthanide centre were to be protected effectively against C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond approach, whilst ensuring a high polarity of the Ln–N σ-bond together with a sufficiently acidic amino proton.
C bond approach, whilst ensuring a high polarity of the Ln–N σ-bond together with a sufficiently acidic amino proton.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkage toward nucleophilic amido attack in the intramolecular hydroamination of a prototypical 1,3-disubstituted
C linkage toward nucleophilic amido attack in the intramolecular hydroamination of a prototypical 1,3-disubstituted ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond approach, whilst ensuring a high polarity of the Ln–N σ-bond together with a sufficiently acidic
C bond approach, whilst ensuring a high polarity of the Ln–N σ-bond together with a sufficiently acidic 

 Please wait while we load your content...
Please wait while we load your content...