Synthesis of hybrid transition-metalloproteinsviathiol-selective covalent anchoring of Rh-phosphine and Ru-phenanthroline complexes†
Abstract
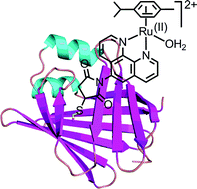
The preparation of hybrid transition
- This article is part of the themed collection: Bridging the gap in catalysis via multidisciplinary approaches

 Please wait while we load your content...
Please wait while we load your content...