How to elucidate and control the redox sequence in vinylbenzoate and vinylpyridine bridged diruthenium complexes†
Abstract
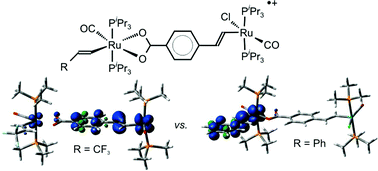
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)(CO)(PiPr3)2Ru(μ-4-OOCC6H4–CH
CH)(CO)(PiPr3)2Ru(μ-4-OOCC6H4–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)RuCl(CO)(PiPr3)2 (R = Ph, 3a or CF3, 3b) and
CH)RuCl(CO)(PiPr3)2 (R = Ph, 3a or CF3, 3b) and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)RuCl(CO)(PiPr3)2 (3c) have been prepared from their
CH)RuCl(CO)(PiPr3)2 (3c) have been prepared from their

 Please wait while we load your content...
Please wait while we load your content...