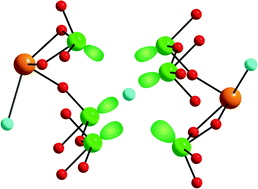
A new layered bismuth tellurium oxychloride, Bi3Te4O10Cl5 has been synthesized by a solid-state reaction under vacuum using Bi2O3, TeO2, and TeCl4 as reagents. The structure of Bi3Te4O10Cl5 has been determined by single-crystal X-ray diffraction. Bi3Te4O10Cl5 contains two different lone pair cations, Bi3+ and Te4+ that are in asymmetric coordination environments. Bi3Te4O10Cl5 has a novel two-dimensional layered structure consisting of distorted BiO4Cl2 octahedra, BiO4Cl3 polyhedra, TeO4 polyhedra, and isolated Cl− ions. Complete structural analysis, infrared spectrum, thermogravimetric analysis, and dipole moment calculations are presented. Crystal data: Bi3Te4O10Cl5, monoclinic, space group C2/m (No. 12), a = 15.372(3) Å, b = 4.1004(7) Å, c = 13.301(2) Å, β = 98.336(5)°, V = 829.5(2) Å3, and Z = 2.

 Please wait while we load your content...
Please wait while we load your content...