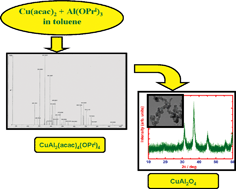
CuAl2(acac)4(OiPr)4 was obtained by the reaction driven by the ligand rearrangement between anhydrous Cu(acac)2 and Al(OPri)3 in toluene under refluxing conditions. The single molecular nature and the stability of the precursor were evidenced by the presence of the molecular ion peak at m/z 749 in the EI mass spectrum. The FT-IR spectrum also confirmed the formation of the molecular precursor with strong bands appearing at 961, 1021, 1289, 1394, 1527 and 1594 cm−1. Very low solubility as well as low stability of the precursor in common solvents hindered the growth of single crystals. The stability could be improved in acetic acid medium which was further used for the controlled hydrolysis of the precursor. A blue gel obtained on hydrolysis for 11 days showed the presence of acetate, isopropoxy and acac moieties in addition to the hydroxide group in the FT-IR spectrum. Fast hydrolysis assisted by ultrasonication with 1.5 ml of the hydrolyzing agent for 6 h resulted in a blue colored gel, the FT-IR spectrum of which also indicated the presence of acetate, isopropoxy, acac and hydroxide moieties. Both the gels showed a mass loss up to 78% according to thermal analysis in air up to 900 °C. While the PXRD pattern of the gel from the controlled hydrolysis yielded monophasic cubic CuAl2O4 on heating at 900 °C in air for 12 h, phase pure product could be obtained within 12 h at 700 °C from the sonicated gel. Both the oxides were nanosized as observed in the TEM images. The particle size distribution obtained from the laser light scattering method showed monodispersity. The room-temperature Raman spectrum of CuAl2O4 exhibited broad bands at 476, 505, 610, 712, 792 cm−1 typical of nanosized crystallites and were assigned based on group theoretical analysis. The 27Al NMR spectrum of CuAl2O4 showed a sharp and intense resonance signal at around δ = 0.112 ppm characteristic of aluminium in octahedral coordination and one sharp signal at δ = 69 ppm corresponding to tetrahedrally coordinated aluminium. Heterogeneous catalytic reduction of p-nitrophenol using CuAl2O4 was followed by UV/visible spectroscopy. CuAl2O4 from the present procedure proved to be an effective catalyst for the reduction of p-nitrophenol.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...