The structure of tavorite LiFePO4(OH) from diffraction and GGA + U studies and its preliminary electrochemical characterization
Abstract
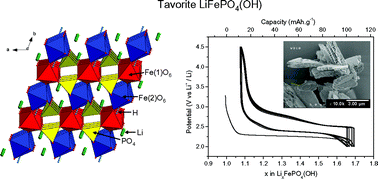
Pure tavorite LiFePO4(OH) was synthesized through a hydrothermal route. A fine structural analysis was done by X-ray and

 Please wait while we load your content...
Please wait while we load your content...