Reduction of N,N′-di(2,6-diisopropylphenyl)carbodiimide (DippNCNDipp) by [SmL2(thf)2] (1) (L = N,N′-bis(2,6-diisopropylphenyl)formamidinate, DippNC(H)NDipp) in PhMe gave [Sm(L)3] (2) in good yield. An analogous reaction of 1 with N,N′-dimesitylcarbodiimide (MesNCNMes) gave [SmL2(MesNC(H)NMes)] (3). In contrast, reduction of N,N′-dicyclohexylcarbodiimide (CyNCNCy) by 1 in PhMe gave mixture of products from which [SmL2(CyNC(CH2Ph)NCy)] (4) and [SmL2(CyNC(H)NCy)] (5) were isolated by fractional crystallisation. Using thf as the reaction solvent, solely compound 5 was crystallised. Reactions of [Sm(L)2(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(thf)] (6) with the carbodiimides RNCNR (R = Cy, Mes) gave [Sm(L)2(RNC(C
CPh)(thf)] (6) with the carbodiimides RNCNR (R = Cy, Mes) gave [Sm(L)2(RNC(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)NR)] (R = Cy (7) or Mes (8)) which are analogues of 4. No reaction was observed between 6 and DippNCNDipp.
CPh)NR)] (R = Cy (7) or Mes (8)) which are analogues of 4. No reaction was observed between 6 and DippNCNDipp.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(thf)]
CPh)(thf)]![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)NR)
CPh)NR)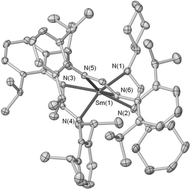

 Please wait while we load your content...
Please wait while we load your content...