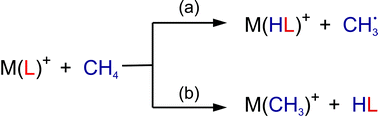
Gas-phase ion–molecule reactions, complemented by DFT and wave-function based electronic structure calculations, are presented, which permit rather detailed descriptions of two fundamental processes related to the activation of methane at room temperature. (i) Recent examples are discussed, which shed light on the role of oxygen-centered radicals in the first step of the oxidative dimerization of methane, i.e. the homolytic C–H bond cleavage of methane. (ii) Thermal ligand exchange processes of the type ML+ + CH4→ M(CH3)+ + LH are analyzed in detail with an emphasis on L = H and F attached to various transition-metal cations M+. It will be demonstrated inter alia that similar systems, e.g. MH+ (M = Ni, Pd, Pt) in their room-temperature reactions with methane, actually exhibit fundamentally different mechanistic scenarios.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...