Synthesis, oxidation chemistry and cytotoxicity studies on ferrocene derivatives of diethylstilbestrol†
Abstract
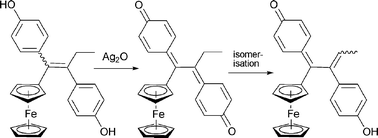
A series of compounds is described in which one of the
- This article is part of the themed collection: Metal anticancer compounds

 Please wait while we load your content...
Please wait while we load your content...