Organic-soluble transition metal-substituted Dawson compounds [(n-C4H9)4N]9[P2W17O61M(Br)] (Mn+ = Co2+, Ni2+, Cu2+ and Zn2+), [(n-C4H9)4N]7[HP2W17O61M(Br)] (Mn+ = Cr3+, Mn3+ and Fe3+) and [K/(n-C4H9)4N]10−n[P2W17O61M(H2O)] (Mn+ = Ir4+, Ru3+ and Pd2+) have been investigated as oxygen transfer agents for H2O2 to a series of primary allylic alcohols to generate epoxides under biphasic reaction conditions (1,2-dichloroethane/H2O) at 30 or 35 °C, such that the effect of variations in the substituted transition metals could be evaluated. The allylic alcohols involved the species R1R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R3)CH2–OH (where R1, R2 and R3 = H or Me), as well as cyclic (2-cyclohexen-1-ol), bicyclic [(R-)-(−)-myrtenol and (R-)-(−)-nopol] and species with two unsaturated sites (geraniol and nerol). The reactions are highly chemoselective and regioselective. The order of reactivity for the M(II)-substituted species is Pd(II) > Zn(II) > Co(II) > Ni(II), and for M(III) and M(IV) substitution is Mn(III) ∼ Ir(IV) > Fe(III) > Cr(III). The observed orders are consistent with the formation of metal(n+)-alcohol species as part of the reaction mechanism. For the more polarizing Ir(IV), however, Ir(IV)-alcoholate species are likely involved in the mechanism. Formation constants for the Mn(III) and Co(II)-phosphopolyoxotungstate-alcohol species with all of the above alcohols have been evaluated in 1,2-dichloroethane at 25 °C and range from 19.0-3.5 M−1. The most likely transition state involves coordination of the alcohol to the transition metal substituted at the lacunary site, or alkoxide in the case of Ir(IV), along with interaction of the double bond of the alcohol with a peroxo group located at a W(VI) site adjacent to the substituted transition metal.
C(R3)CH2–OH (where R1, R2 and R3 = H or Me), as well as cyclic (2-cyclohexen-1-ol), bicyclic [(R-)-(−)-myrtenol and (R-)-(−)-nopol] and species with two unsaturated sites (geraniol and nerol). The reactions are highly chemoselective and regioselective. The order of reactivity for the M(II)-substituted species is Pd(II) > Zn(II) > Co(II) > Ni(II), and for M(III) and M(IV) substitution is Mn(III) ∼ Ir(IV) > Fe(III) > Cr(III). The observed orders are consistent with the formation of metal(n+)-alcohol species as part of the reaction mechanism. For the more polarizing Ir(IV), however, Ir(IV)-alcoholate species are likely involved in the mechanism. Formation constants for the Mn(III) and Co(II)-phosphopolyoxotungstate-alcohol species with all of the above alcohols have been evaluated in 1,2-dichloroethane at 25 °C and range from 19.0-3.5 M−1. The most likely transition state involves coordination of the alcohol to the transition metal substituted at the lacunary site, or alkoxide in the case of Ir(IV), along with interaction of the double bond of the alcohol with a peroxo group located at a W(VI) site adjacent to the substituted transition metal.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(R3)CH2–
C(R3)CH2–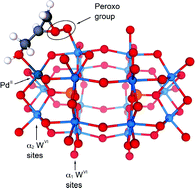

 Please wait while we load your content...
Please wait while we load your content...