Binary and ternary lanthanide centered hybrid polymeric materials: coordination bonding construction, characterization, microstructure and photoluminescence†
Abstract
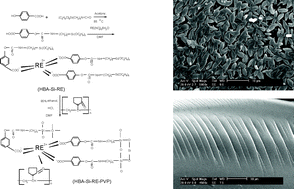
A functional molecular bridge (named as HBA-TEPIC) (HBA = 1,4-hydroxybenzoic acid, TEPIC =

 Please wait while we load your content...
Please wait while we load your content...