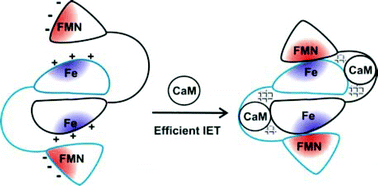
There is still much that is unknown about how nitric oxide (NO) biosynthesis by NO synthase (NOS) isoform is tightly regulated at the molecular level. This is remarkable because deviated NO production in vivo has been implicated in an increasing number of diseases that currently lack effective treatments, including stroke and cancer. Given the significant public health burden of these diseases, the NOS enzyme family is a key target for development of new pharmaceuticals. Three NOS isoforms, inducible, endothelial and neuronal NOS (iNOS, eNOS and nNOS, respectively), achieve their key biological functions via stringent regulations of interdomain electron transfer (IET) processes. Unlike iNOS, eNOS and nNOS isoforms are controlled by calmodulin (CaM) binding through facilitating catalytically significant IET processes. The CaM-modulated NOS output state is an IET-competent complex between the flavin mononucleotide (FMN) domain and the catalytic heme domain. The output state facilitates the catalytically essential FMN–heme IET, and thereby enables NO production by NOS. Due to lack of reliable techniques for specifically determining the inter-domain FMN–heme interactions and their direct effects on the catalytic heme center, the molecular mechanism that underlies the output state formation remains elusive. The recent developments in our understanding of mechanisms of the NOS output state formation that are driven by a combination of molecular biology, laser flash photolysis, and spectroscopic techniques are the subject of this perspective.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...