The structural effects of the Cys-S-S-Cys bridge exchange by the His-Cu(II)-His motif studied on natural peptides — a promising tool for natural compounds-based design†
Abstract
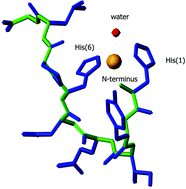
A replacement of both Cys residues by His in
* Corresponding authors
a
Department of Inorganic Chemistry, Wrocław Medical University, Szewska 38, Wrocław, Poland
E-mail:
jbrasun@chnorg.am.wroc.pl
b Faculty of Chemistry, University of Wrocław, F. Joliot- Curie 14, Wrocław, Poland
c Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Pawińskiego 5a, Warsaw, Poland
d College of Inter-Faculty Individual Studies In Mathematics and Natural Sciences, Warsaw University, ul. Żwirki i Wigury 93, Warsaw, Poland
e Slovenian NMR Center, National Institute of Chemistry, Hajdrihova 19, Ljubljana, Slovenia
A replacement of both Cys residues by His in

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. Brasuń, M. Cebrat, Ł. Jaremko, M. Jaremko, G. Ilc, O. Gładysz and I. Zhukov, Dalton Trans., 2009, 4853 DOI: 10.1039/B901676G
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
