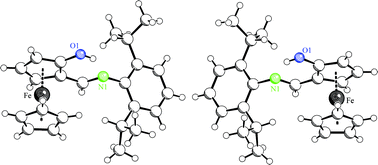
Efficient selective synthetic pathways to the O-(tert-butyl)diphenylsilyl-protected 2-hydroxyferrocene carbaldehyde enantiomers [(p-S)-12 and (p-R)-12, each >99.5% ee] are described. The general synthesis starts from Kagan's ferrocene carbaldehyde acetal [(S,S)-6], bearing the (2S,4S)-[4-(methoxymethyl)-1,3-dioxan-2-yl] chiral auxiliary. The synthetic route to (p-S)-12 involves a sequence of directed ortho-lithiation/iodination, followed by acetoxylation (Cu2O/acetic acid), saponification and protection by the TBDPS silyl-protective group. Acid-catalyzed hydrolysis of the chiral acetal then gave the O-TBDPS-protected (p-S)-configurated hydroxyferrocene carbaldehyde enantiomer in an overall yield of 76% over 4 steps. The (p-R)-12 enantiomer was prepared by a similar route starting from (S,S)-6 using the –SiMe3group as a reversible blocking group to direct the required functionalization to the (p-R)-series. A total of six steps furnished (p-R)-12 in a combined yield of 27%. The aldehyde enantiomers of 12 were used for the synthesis of the corresponding N-2,6-diisopropylphenyl-imines. Subsequent deprotection gave the new “ferrocene-salimine” ligands 2-hydroxy-[N-(2,6-diisopropylphenyl)iminomethyl]-ferrocene (p-S)-2a and (p-R)-2a, respectively. We also synthesized the (p-S)-enantiomers of the corresponding N-mesityl and N-pentafluorophenyl aldimine derivatives [(p-S)-2b, (p-S)-2c].
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...