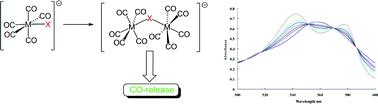
The CO-releasing ability of a diverse library of primary metal carbonyl complexes has been assessed using a deoxymyoglobin-carbonmonoxymyglobin assay. A wide spectrum of rates for the CO-release process was observed in aqueous systems. For octahedral d6 complexes, the rate was found to decrease in the sequence FeI2(CO)4 > [NEt4][V(CO)6] > MnBr(CO)5 > Cr(CO)6 implying that CO-release is not controlled by the metal–carbon bond strengths. Within the series, [NEt4][MX(CO)5] (M = Cr, Mo, W; X =Cl, Br, I), the rate of CO-release was found to decrease down the group (Cr > Mo > W), whilst within the chromium series a similar trend was observed for the halide (Cl > Br > I). The d4 complexes [NEt4][MI3(CO)4] (M = Mo, W) exhibit faster release than their d6 congeners. A mechanistic investigation into the [NEt4][MX(CO)5] series revealed the intermediacy of [{M(CO)5}2(μ-X)]− in the CO-release process and that the hydrolysis of the M–X bond, rather than the intrinsic strength of M–CO bonds, controls the rate of CO-release in aqueous systems.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...