Extended structures containing Pt(ii)–Tl(i) bonds. Effect of these interactions on the luminescence of cyclometalated Pt(ii) compounds†
Abstract
Neutralization reactions of the appropriate precursors (NBu4)[Pt(bzq)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–R)2] and (NBu4)[Pt(C⁁N)(CN)2] (C⁁N =
C–R)2] and (NBu4)[Pt(C⁁N)(CN)2] (C⁁N = ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–R)2}2] [R = Ph (1), C5H4N-2 (2)] and [PtTl(C⁁N)(CN)2] [C⁁N =
C–R)2}2] [R = Ph (1), C5H4N-2 (2)] and [PtTl(C⁁N)(CN)2] [C⁁N = ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) nonclassical interactions. Compounds 3 and 4 show extended 2-D networks by connection of the organometallic “PtTl(C⁁N)(CN)2” units, through secondary Tl⋯N
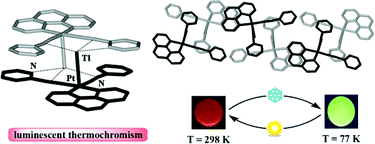
C) nonclassical interactions. Compounds 3 and 4 show extended 2-D networks by connection of the organometallic “PtTl(C⁁N)(CN)2” units, through secondary Tl⋯N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C contacts and moderate π⋯π(bzq) interactions in the case of 3. Complexes 1–3 containing the bzq group exhibit in the solid state “luminescence thermochromism” associated to dual emission. At room temperature they show an intense, visible orange (1: λmax 625 nm), orange-red (2: λmax 640 nm) or yellow (3: λmax 582 nm) luminescence that changes to yellowish-green (1: λmax 532 nm) or green [2: λmax 524 nm; 3: λmax 512 nm] upon cooling to 77 K. The unstructured low energy (LE) bands attributed to 3π–π* excimeric emissions due to extensive π–π interactions are dominant at room temperature. By contrast, the high energy (HE) bands are highly structured and predominant at 77 K. Due to the presence of Pt–Tl bonds these HE emissions are bathochromically shifted in relation to the precursors′ ones and have been tentatively assigned to a metal-metal′-to-
C contacts and moderate π⋯π(bzq) interactions in the case of 3. Complexes 1–3 containing the bzq group exhibit in the solid state “luminescence thermochromism” associated to dual emission. At room temperature they show an intense, visible orange (1: λmax 625 nm), orange-red (2: λmax 640 nm) or yellow (3: λmax 582 nm) luminescence that changes to yellowish-green (1: λmax 532 nm) or green [2: λmax 524 nm; 3: λmax 512 nm] upon cooling to 77 K. The unstructured low energy (LE) bands attributed to 3π–π* excimeric emissions due to extensive π–π interactions are dominant at room temperature. By contrast, the high energy (HE) bands are highly structured and predominant at 77 K. Due to the presence of Pt–Tl bonds these HE emissions are bathochromically shifted in relation to the precursors′ ones and have been tentatively assigned to a metal-metal′-to-

 Please wait while we load your content...
Please wait while we load your content...