Synthesis and characterization of a series of manganese phosphonate complexes with various valences and nuclearity†
Abstract
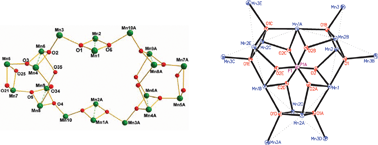
A series of new manganese phosphonate clusters with unprecedented topologies, [Mn20Na4O12(t-BuPO3)12(O2CMe)16(H2O)12]·5H2O (1), [Mn15O6(MePO3)2(MeCOO)18(H2O)12][MePO3H]2·12H2O (2), and [Mn(t-BuPO3H)2(phen)2]·MeCOOH (3) have been synthesized and characterized. The core of 1 is composed of two folded “butterfly-like” [Mn4(μ3-O)2] units and two trigonal prism [Mn6O6] units connected by four μ3-O, resulting in a twisted oval of twenty homovalent manganese(III) ions. The pentadecanuclear core of complex 2 is mixed-valent, containing six MnIII and nine MnII ions and features six μ4-O and two capping MePO32− anions adopting the binding mode [6.222], forming a rudder-like cage structure with a chiral D3 symmetry. Compound 3 is a manganese(II) phosphonate and features a 1D chain configuration packed by hydrogen bonds and π–π stacking interactions. Magnetic susceptibility measurements reveal that compounds 1, 2 and 3 display antiferromagnetic interactions between the adjacent manganese ions, while the in-phase signal χ′MT and out-of-phase signal χ″M of complex 1 exhibit frequency-dependence below approximately 3 K, but no frequency-dependence is observed in the ac signals of complex 2.

 Please wait while we load your content...
Please wait while we load your content...