Coordination chemistry of bisstannylenes with platinum(0)†
Abstract
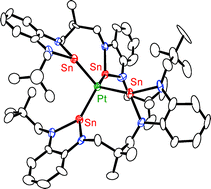
Reaction of stoichiometric amounts of the benzannulated bisstannylene 1 with [Pt(nbe)3] in the presence of PPh3 and OPPh3 gave complex 3 with an Sn2PtP2 core and an O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3 molecule coordinated to one of the stannylene donors according to an X-ray diffraction study. Complex 3 was also obtained by substitution of two PPh3
PPh3 molecule coordinated to one of the stannylene donors according to an X-ray diffraction study. Complex 3 was also obtained by substitution of two PPh3

 Please wait while we load your content...
Please wait while we load your content...