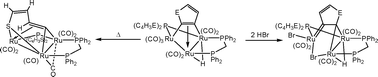
The synthesis and reactivity of the thiophyne and furyne clusters [Ru3(CO)7(μ-dppm)(μ3-η2-C4H2E){μ-P(C4H3E)2}(μ-H)] (E = S, O) is reported. Addition of P(C4H3E)3 to [Ru3(CO)10(μ-dppm)] (1) at room temperature in the presence of Me3NO gives simple substitution products [Ru3(CO)9(μ-dppm){P(C4H3E)3}] (E = S, 2; E = O, 3). Mild thermolysis in the presence of further Me3NO affords the thiophyne and furyne complexes [Ru3(CO)7(μ-dppm)(μ3-η2-C4H2E){μ-P(C4H3E)2}(μ-H)] (E = S, 4; E = O, 6) resulting from both carbon-hydrogen and carbon-phosphorus bond activation. In each the C4H2E (E = S, O) ligand donates 4-electrons to the cluster and the rings are tilted with respect to the μ-dppm and the phosphido-bridged open triruthenium unit. Heating 4 at 80 °C leads to the formation of the ring-opened cluster [Ru3(CO)5(μ-CO)(μ-dppm)(μ3-η3-SC4H3){μ-P(C4H3S)2}] (5) resulting from carbon-sulfur bond scission and carbon-hydrogen bond formation and containing a ring-opened μ3-η3-1-thia-1,3-butadiene ligand. In contrast, a similar thermolysis of 3 affords the phosphinidene cluster [Ru3(CO)7(μ-dppm)(μ3-η2-C4H2O){μ3-P(C4H3O)}] (7) resulting from a second phosphorus-carbon bond cleavage and (presumably) elimination of furan. Treatment of 4 and 6 with PPh3 affords the simple phosphine-substituted products [Ru3(CO)6(PPh3)(μ-dppm)(μ3-η2-C4H2E){μ-P(C4H3E)2}(μ-H)] (E = S, 8; E = O, 9). Both thiophyne and furyne clusters 4 and 6 readily react with hydrogen bromide to give [Ru3(CO)6Br(μ-Br)(μ-dppm)(μ3-η2-η1-C4H2E){μ-P(C4H3E)2}(μ-H)] (E = S, 10; E = O, 11) containing both terminal and bridging bromides. Here the alkynes bind in a highly unsymmetrical manner with one carbon acting as a bridging alkylidene and the second as a terminally bonded Fisher carbene. As far as we are aware, this binding mode has only previously been noted in ynamine complexes or those with metals in different oxidation states. The crystal structures of seven of these new triruthenium clusters have been carried out, allowing a detailed analysis of the relative orientations of coordinated ligands.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...