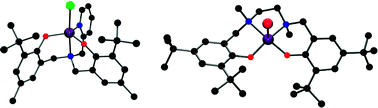
The structures and properties of six new iron(III) diamine-bis(phenolate) complexes are reported. Reaction of anhydrous FeX3 salts (where X = Cl or Br) with the diprotonated tripodal tetradentate ligands 2-pyridylamino-N,N-bis(2-methylene-4-methyl-6-tert-butylphenol), H2[L1], and N,N-dimethyl-N′,N′-bis(2-methylene-4-methyl-6-tert-butylphenol)ethylenediamine, H2[L2], produces the trigonal bipyramidal iron(III) complexes, [L1]FeCl 1, [L1]FeBr 2, [L2]FeCl 3 and [L2]FeBr 4. Reaction of FeX3 with the related linear tetradentate ligand N,N′-bis(4,6-tert-butyl-2-methylphenol)-N,N′-bismethyl-1,2-diaminoethane, H2[L3], generates square pyramidal iron(III) complexes, [L3]FeCl 5 and [L3]FeBr 6. Complexes 1–6 have been characterized using electronic absorption spectroscopy and magnetometry. Single crystal X-ray molecular structures have been determined for complexes 1, 3, 5 and 6.

 Please wait while we load your content...
Please wait while we load your content...