A series of trifluoromethanesulfonate (OTf) salts of N-heterocyclic phospheniums (NHP) bearing phenyl (1a), para-methoxyphenyl (1b), 2,6-diisopropylphenyl (1c) and mesityl (1d) substituents is reported. The compounds 1b–d are made by a modification to a literature procedure that improves the overall yields for 1c and 1d by 15 and 23%, respectively. Two unwanted side-products in the synthesis of 1d, the diammonium salt, [(2,6-iPr-C6H3)N(H)2CH2CH2N(H)2(2,6-iPr-C6H3)]Cl2 (4) and the bisphosphine (2,6-iPr-C6H3)N(PCl2)CH2CH2N(PCl2)(2,6-iPr-C6H3) (5), are crystallographically characterized, as is the intermediate cyclic chlorophosphine, C1P![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) N(4-OMe-C6H4)CH2CH2N
N(4-OMe-C6H4)CH2CH2N![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (4-OMe-C6H4) (3b). The phenyl-substituted NHP 1a is fully characterized, including by X-ray crystallography, for the first time; this compound contains a short P–O contact of 2.1850(14) Å. Cycloaddition reactions of 1a–d with 2,3-dimethyl-1,3-butadiene give the expected spirocyclic phospholeniums, 7,8-dimethyl-1,4-diaryl-1,4-diaza-5-phopshoniaspiro[4.4]non-7-ene, as their OTf salts (6a–d), while reactions with N,N′-dimesityl-1,4-diaza-1,3-butadiene give, except in the case of 1c, which is too bulky to react, the aza analogues, 1,4-dimesityl-6,9-diaryl-1,4,6,9-tetraaza-5-phosphoniaspiro[4.4]non-2-ene (7a, 7b and 7d). The sterically congested 7d is in thermal equilibrium with 1d and free diazadiene, and undergoes a substitution reaction with 2,3-dimethyl-1,3-butadiene to give 6d.
(4-OMe-C6H4) (3b). The phenyl-substituted NHP 1a is fully characterized, including by X-ray crystallography, for the first time; this compound contains a short P–O contact of 2.1850(14) Å. Cycloaddition reactions of 1a–d with 2,3-dimethyl-1,3-butadiene give the expected spirocyclic phospholeniums, 7,8-dimethyl-1,4-diaryl-1,4-diaza-5-phopshoniaspiro[4.4]non-7-ene, as their OTf salts (6a–d), while reactions with N,N′-dimesityl-1,4-diaza-1,3-butadiene give, except in the case of 1c, which is too bulky to react, the aza analogues, 1,4-dimesityl-6,9-diaryl-1,4,6,9-tetraaza-5-phosphoniaspiro[4.4]non-2-ene (7a, 7b and 7d). The sterically congested 7d is in thermal equilibrium with 1d and free diazadiene, and undergoes a substitution reaction with 2,3-dimethyl-1,3-butadiene to give 6d.
![[upper bond 1 start]](https://www.rsc.org/images/entities/char_e010.gif) N(4-OMe-C6H4)CH2CH2N
N(4-OMe-C6H4)CH2CH2N![[upper bond 1 end]](https://www.rsc.org/images/entities/char_e011.gif) (4-OMe-C6H4) (3b). The
(4-OMe-C6H4) (3b). The 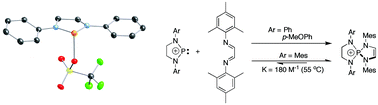

 Please wait while we load your content...
Please wait while we load your content...