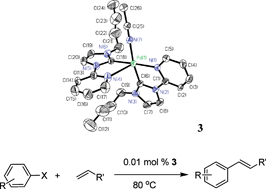
[Pd(L1)2(CH3CN)](PF6)2 (L1 = 1-n-butyl-3-(2-pyrimidyl)imidazolylidene, 3) and [Pd(L2)2](PF6)2 (L2 = 1-(2-picolyl)-3-(2-pyrimidyl)imidazolylidene 4), prepared viacarbene transfer reactions of [Ag(L1)2]PF6 (1) and [Ag2(L2)2](PF6)2 (2) with palladium salts, respectively, have been fully characterized by 1H and 13C NMR spectroscopy and elemental analysis. The X-ray crystal structures of complexes 1–4 are reported. Complex 3 is an unusual pentacoordinated palladium complex, in which the palladium is coordinated by two imidazolylidene, two pyridine, and one acetonitrile molecule in a square-pyramidal geometry. The apical position is occupied by a pyrimidine nitrogen atom with a relatively long Pd–N distance (2.762(6) Å). Complex 4 is a typical square-planar palladium complex with palladium surrounded by two pairs of cis-arranged pyridine and imidazolylidene ligands. The complexes exhibit good catalytic activities in the Heck coupling reaction of aryl bromides and activated aryl chlorides under mild conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...