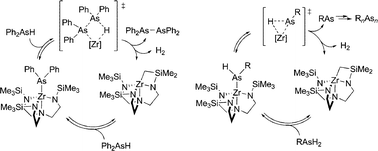
Triamidoamine-supported zirconium complexes have been demonstrated to catalyze a range of bond-forming events utilizing arsines. Three different mechanisms have been observed in these reactions. In the first mechanism, triamidoamine-supported zirconium complexes of the general type (N3N)ZrX (N3N = N(CH2CH2NSiMe3)33−; X = monoanionic ligand) catalyzed the dehydrogenative dimerization of diphenylarsine. Mechanistic analysis revealed that As–As bond formation proceeds via σ-bond metathesis steps similar to the previously reported dehydrocoupling of phosphines by the same catalysts. In the second mechanism, sterically encumbered primary arsines appear to be dehydrocoupled via α elimination of an arsinidene fragment. Dehydrocoupling of dmpAsH2 (dmp = 2,6-dimesitylphenyl) to form (dmp)As = As(dmp) by (N3N)Zr-complexes appeared to proceed via elimination of dmpAs: from the arsenido intermediate, (N3N)ZrAsH(dmp). Further support for α-arsinidene elimination came from the thermal decomposition of (N3N)ZrAsHMes (9) to (MesAs)4 (10), which obeyed first-order kinetics. In the third mechanism, the observation of stoichiometric insertion reactivity of the Zr–As bond with polar substrates, PhCH2NC, PhCN, (1-napthyl)NCS, and CS2, led to the development of intermolecular hydroarsination catalysis of terminal alkynes. Here, (N3N)ZrAsPh2 (2) catalyzed the addition of diphenylarsine to phenylacetylene and 1-hexyne to give the respective vinylarsine products. Arsenido complexes 2 and 9 and tetraarsine 10 have been structurally characterized.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...