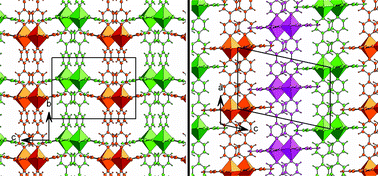
We report the synthesis of five coordination polymers of divalent transition metals combined with 5-hydroxyisophthalic acid (HIP) and 4,4′-bipyridyl (bipy). Mn forms two polytypic two-dimensional coordination polymers, Mn(HIP)(bipy)·3H2O and Mn(HIP)(bipy)·¼bipy·2H2O (I and II, with ABAB and ABC stacking sequences, respectively); Ni forms a chiral hexagonal three-dimensional coordination polymer with two interpenetrating trigonal sublattices exhibiting the “dual quartz” topology, Ni(HIP)(bipy)(H2O) (III); Cu forms a one-dimensional coordination polymer containing arrays of infinite hydrogen-bonded molecular ribbons, Cu(HIP)2(bipy) (IV); and Zn forms a two-dimensional coordination polymer with a stair-stepped layered structure, Zn2(HIP)2(bipy)(H2O)2·H2O (V). The M–HIP–M connections are perpendicular to the M–bipy–M connections in all structures where they are present.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...