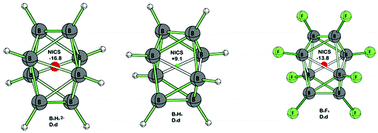
The symmetry constrained geometries of the eight- and nine-vertex polyhedral boranes and haloboranes BnXnz (n = 8 and 9; X = H, F and Cl; z = −2, −1 and 0) were optimized at the B3LYP/6-311+G(d) level and their nucleus-independent chemical shifts (NICS) were calculated using the GIAO method with Kohn–Sham orbitals. Substitution of halogens on borane cages was found to significantly impact not only the geometric but also magnetic properties. Multiple fluorine substituents cause a deviation from the Wade–Mingos skeletal electron rules in B8F82−, resulting in a distortion from the expected D2d bisdisphenoid to a C2vnido type bicapped trigonal prism. However, all of the nine-vertex cages B9X9z retain the D3h tricapped trigonal prismatic structure of B9H92−. The presence of halogen substituents was found to enhance the three-dimensional diatropic ring currents within the dianionic borane cages B8X82− and B9X92−. For the neutral structures the NICS values indicate BnFn to be aromatic, BnCln to be essentially non-aromatic, and BnHn to be antiaromatic (n = 8, 9).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...