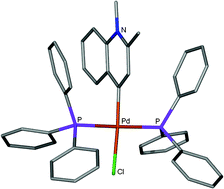
A whole library of six-membered N-heterocyclic carbene complexes of Ni(II) and Pd(II) were prepared by oxidative substitution. In some of these compounds the heteroatom occurs in a position distant from the carbene donor atom. Combined structural and physical data, especially 13C NMR results, indicate carbene character in such ligands. DFT quantum mechanical calculation at the RI-BP56/SVP level allowed the extraction of valuable chemical information predicting that rNHC (r = remote) ligands would bond more strongly than their nNHC (n = normal) isomers. This result is also corroborated by calculations on the metal complexes themselves. Orbital overlap (mainly σ) follows the order N2HC5 < nN1HC6 < rN1HC6 when ligands derived from halo-imidazolium and halo-pyridinium salts are compared. In C–C coupling catalysis using Pd(II) and Ni(II) complexes, the simple one-N, six-membered carbene complexes are superior to simple two-N, five-membered examples but clear differentiation between nNHC and rNHC precatalysts in the former family, is not always possible.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...