A structural analysis of {M4O4} cubanes where M = Mn and Fe†
Abstract
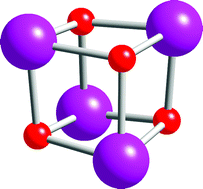
The structures of the M4O4 units found in manganese and iron cubanes are analysed. The model used is that established previously for cobalt and nickel cubanes based on a distortion of the cube by compression of the oxygen atoms along a body diagonal. Further distortion which maintains a S4 or, less frequently, a C3 axis is generally seen. In spite of the distortion, average M–M distances in a cubane are quite constant for a given oxidation state and generally decrease as the metal is oxidised. The angles at the oxygen atoms increase from 90° for the ideal cube to around 97°. For metal oxidation states above or equal to +IIIµ3-hydroxo

 Please wait while we load your content...
Please wait while we load your content...