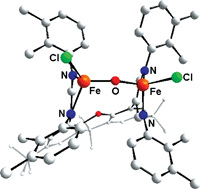
With the background that certain natural systems utilize two FeII centres in their prosthetic groups for the activation of O2, a ligand system containing two parallel β-diiminato binding sites linked by a xanthene backbone ([RXanthdim]2− with residues R = 2,3-dimethylphenyl and 2,4-difluorophenyl at the iminato units, respectively) was investigated with respect to its FeII coordination chemistry in order to study O2activation reactions. Hence, the corresponding lithium salts were treated with FeCl2 to yield the complexes [Me2C6H3Xanthdim]Fe2Cl3(Li(thf)3), 1 and [F2C6H3Xanthdim]Fe2Cl3(Li(thf)3), 2, respectively, each of which comprises Cl–Fe(µ-Cl)Fe–Cl(Li(thf)3) units. 1 and 2 indeed readily react with O2 to give the oxides[RXanthdim]Fe2Cl2O containing FeIII–O–FeIII moieties. Due to the electron withdrawing F atoms 2 reacts more slowly than 1. The molecular structures of 1, 2 and [Me2C6H3Xanthdim]Fe2Cl2O, 3, as determined by single crystal X-ray diffraction are discussed, and an investigation concerning their magnetic properties revealed an antiferromagnetic coupling of the two iron centres in all complexes; naturally, the strongest coupling is observed for 3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...