Molecular tectonics: design and generation of charge-assisted, H-bonded, hybrid molecular networks based on amidinium cations and thio- or isothio-cyanatometallates†
Abstract
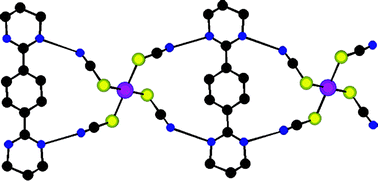
Upon combining the bis-amidinium dication 1-2H+ with thiocyanatometallate M(SCN)42− (M = Pd, Hg) or isothiocyanatometallate Cu(NCS)42− anions (behaving as H-bond donor and acceptors, respectively) three new hybrid molecular networks have been obtained in the crystalline phase and structurally characterized by

 Please wait while we load your content...
Please wait while we load your content...