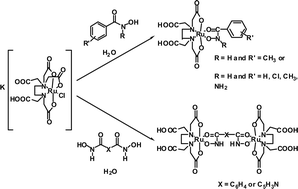
The synthesis and spectroscopic characterisation of novel mononuclear RuIII(edta)(hydroxamato) complexes of general formula [Ru(H2edta)(monoha)] (where monoha = 3- or 4-NH2, 2-, 3- or 4-Cl and 3-Me-phenylhydroxamato), as well as the first example of a RuIII-N-aryl aromatic hydroxamate, [Ru(H2edta)(N-Me-bha)]·H2O (N-Me-bha = N-methylbenzohydroxamato) are reported. Three dinuclear RuIII complexes with bridging dihydroxamato ligands of general formula [{Ru(H2edta)}2(μ-diha)] where diha = 2,6-pyridinedihydroxamato and 1,3- or 1,4-benzodihydroxamato, the first of their kind with RuIII, are also described. The speciation of all of these systems (with the exception of the Ru–1,4-benzodihydroxamic acid and Ru–N-methylbenzohydroxamic systems) in aqueous solution was investigated. We previously proposed that nitrosyl abstraction from hydroxamic acids by RuIII involves initial formation of RuIII-hydroxamates. Yet, until now, no data on the rate of nitric oxide (NO) release from hydroxamic acids has been published. We now describe a UV-VIS spectroscopic study, where we monitored the decrease in the ligand-to-metal charge-transfer band of a series of RuIII-monohydroxamates with time, with a view to gaining an insight into the NO-releasing properties of hydroxamic acids.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...