Kinetics and mechanism of the oxidation of water soluble porphyrin FeIIITPPS with hydrogen peroxide and the peroxomonosulfate ion†
Abstract
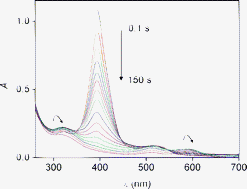
The overall six-electron ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Although no unambiguous proposal for the structure of Int2 can be made, it is most probably the product of the four-electron oxidation of the original FeIIITPPS, contains an iron-oxo center and has a dissociable
O. Although no unambiguous proposal for the structure of Int2 can be made, it is most probably the product of the four-electron oxidation of the original FeIIITPPS, contains an iron-oxo center and has a dissociable

 Please wait while we load your content...
Please wait while we load your content...