Mechanism of reaction of alkyl radicals with (NiIIL)2+ complexes in aqueous solutions
Abstract
The reactions of methyl radicals, ˙CH3, with the macrocyclic complexes NiIIL1–5 (L1–5 = cyclam derivatives, vide infra) and NiIIedta in aqueous solutions were studied. Methyl radicals react with all these nickel complexes, forming intermediates with NiIII–C σ-bonds. The LmNiIII–CH3 complexes are formed in equilibria processes with relatively fast forward rate constants of kf > 1 × 108 M−1 s−1 (except in the case of NiL2-trans I cyclam, where the reaction is slower). In all cases the decomposition of the transient complexes occurs via the homolytic cleavage of the metal–carbon σ-bond. When the homolysis is relatively slow, an isomerisation process of the transient is also observed with the exception of NiL2, where no isomerisation was observed. The results suggest that the strength of the NiIII–CH3 σ-bond is mainly affected by steric hindrance.
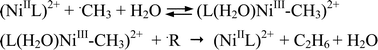

 Please wait while we load your content...
Please wait while we load your content...