Reaction of [Cu2(O2CMe)4(H2O)2] with 2,6-di-(2-pyridylcarbonyl)-pyridine (pyCOpyCOpy or dpcp) in MeCN–H2O 10 : 1, led to the pentanuclear copper(II) complex [Cu5(O2CMe)6{pyC(O)(OH)pyC(O)(OH)py}2] (1) which crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The copper(II) atoms are arranged in an “S”-shaped configuration, and are bridged by the doubly deprotonated bis(gem-diol) form of the ligand, pyC(O)(OH)pyC(O)(OH)py2−. Magnetic susceptibility data indicate the interplay of both ferro- and antiferromagnetic intramolecular interactions stabilizing an S = 3/2 ground state. Fitting of the data according to a next-nearest-neighbour model {Ĥ = −[J1(Ŝ1Ŝ2 + Ŝ1′Ŝ2′) + J2(Ŝ2Ŝ3 + Ŝ3′Ŝ2′) + J3(Ŝ1Ŝ3 + Ŝ3′Ŝ1′) + J4(Ŝ2Ŝ2′)]} yields exchange coupling constants J1 = +39.7 cm−1, J2 = −15.9 cm−1, J3 = −8.3 cm−1 and J4 = +4.3 cm−1, leading to an S = 3/2 ground state. X-Band EPR spectroscopy indicates a zero-field splitting of the ground state with |D3/2| = 0.38 cm−1.
space group. The copper(II) atoms are arranged in an “S”-shaped configuration, and are bridged by the doubly deprotonated bis(gem-diol) form of the ligand, pyC(O)(OH)pyC(O)(OH)py2−. Magnetic susceptibility data indicate the interplay of both ferro- and antiferromagnetic intramolecular interactions stabilizing an S = 3/2 ground state. Fitting of the data according to a next-nearest-neighbour model {Ĥ = −[J1(Ŝ1Ŝ2 + Ŝ1′Ŝ2′) + J2(Ŝ2Ŝ3 + Ŝ3′Ŝ2′) + J3(Ŝ1Ŝ3 + Ŝ3′Ŝ1′) + J4(Ŝ2Ŝ2′)]} yields exchange coupling constants J1 = +39.7 cm−1, J2 = −15.9 cm−1, J3 = −8.3 cm−1 and J4 = +4.3 cm−1, leading to an S = 3/2 ground state. X-Band EPR spectroscopy indicates a zero-field splitting of the ground state with |D3/2| = 0.38 cm−1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The copper(II) atoms are arranged in an “S”-shaped configuration, and are bridged by the doubly deprotonated bis(gem-diol) form of the
space group. The copper(II) atoms are arranged in an “S”-shaped configuration, and are bridged by the doubly deprotonated bis(gem-diol) form of the 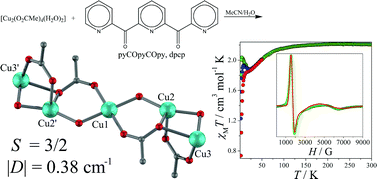

 Please wait while we load your content...
Please wait while we load your content...