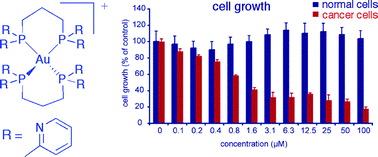
The novel water soluble bidentate phosphine ligand 1,3-bis(di-2-pyridylphosphino)propane (d2pypp) has been synthesized by a convenient route involving treatment of 2-pyridyllithium with Cl2P(CH2)3PCl2 and isolation in crystalline form as the hydrochloride salt. The synthesis of the precursor Cl2P(CH2)3PCl2 has been optimized by the use of triphosgene as the chlorinating agent. The 2 : 1 and 1 : 2 AuCl : d2pypp adducts have been synthesized and characterized by NMR spectroscopy and single crystal X-ray studies, and shown to be of the form (AuCl)2(µ-d2pypp-P,P′) and [Au(d2pypp-P,P′)2]Cl(·3.75H2O), respectively. The latter is more lipophilic than analogous 1 : 2 adducts of gold(I) chloride with the diphosphine ligands 1,2-bis(di-n-pyridylphosphino)ethane (dnpype) for n = 2, 3 and 4, based on measurement of the n-octanol–water partition coefficient (log P = −0.46). A single crystal structure determination of the 1 : 2 Au(I) complex of the 3-pyridyl ethane ligand shows it to be of the form [Au(d3pype-P,P′)2]Cl·5H2O. The in vitro cytotoxic activity of [Au(d2pypp)2]Cl was assessed in human normal and cancer breast cells and selective toxicity to the cancer cells found. The significance of these results to the antitumour properties of chelated 1 : 2 Au(I) diphosphine complexes is discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...