Stereogenic properties of spiranes combined with four equivalent conventional centres of chirality
Abstract
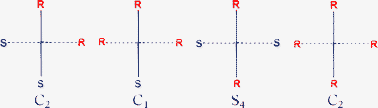
Derivatives of the tri-spirane pentaerythritoxy-cyclophosphazene compound, 1, have been used to investigate the stereogenic properties of spiranes combined with four equivalent conventional centres of chirality. In compound 1 the two inner rings are carbocyclic and symmetrical and the two outer rings are cyclotriphosphazenes substituted in different positions to provide the conventional centres of chirality. The case of combining spiranes with four equivalent centres of chirality has been investigated by the reaction of 1 with

 Please wait while we load your content...
Please wait while we load your content...